A nanodrug for effective treatment of paradoxical inflammation - a promising arm in the fight against Covid-19?
SARS-CoV-2, the virus responsible for Covid-19, causes infection that can lead to acute respiratory distress syndrome with high inflammation in lung cells. A team at the Institut Galien (Université Paris-Saclay, CNRS) has been working for several years on a nanodrug combining several active ingredients to treat uncontrolled secondary inflammatory reactions to bacterial infections. Since the recent SARS-CoV-2 epidemic is also characterized by a paradoxical inflammatory reaction, this multifunctional nanodrug may be an effective treatment of viral infection.
Infections caused by a pathogen - a virus or bacteria, can cause inflammation that triggers the famous "cytokine storm" observed in Covid-19 cases. A cytokine storm releases molecules that encourage inflammation in some cases and reduce it in others. In addition to inflammation, highly reactive molecules, Reactive Oxygen Species (ROS), are produced in the body, causing tissue damage. “This type of inflammatory reaction is a vicious circle," explains Professor Patrick Couvreur from the Institut Galien (Université Paris-Saclay, CNRS). “The inflammatory reaction generates the formation of ROS, which in turn generate damage that activates the inflammatory reaction, and so on.”
Enveloping active ingredients to target cells more effectively
In 2017, Patrick Couvreur and his team began work on a septic-shock model that causes paradoxical inflammations. Researchers injected lipopolysaccharides into laboratory mice to test a new nanodrug. These molecules from the bacterial wall trigger inflammation and the subsequent cytokine storm.
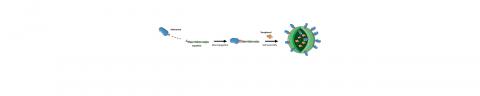
The tested nanodrug contains an anti-inflammatory molecule called adenosine. It is usually considered ineffective to be used as because it is metabolised by the body too quickly. To stabilise it, researchers chemically paired adenosine with squalene, a lipid naturally produced by the human body. Due to the extremely compact molecular structure of squalene, the adenosine-squalene bioconjugate spontaneously organises in nanoparticles. The researchers then added alpha-tocopherol, a type of vitamin E with antioxidant properties that impedes ROS production. Due to its lipophilic nature, alpha-tocopherol can be inserted into adenosine-squalene nanoparticles to form a multifunctional nanodrug with a diameter of approximately 70 nanometres.
After multiple tests on cells and animal models, the team characterised the antioxidant and anti-inflammatory activity of the resulting nanoparticles. “We have shown the effectiveness of nanodrugs on cell cultures which have been subjected to oxidative phenomena or inflammatory shocks. We also observed its effectiveness in mice that have suffered from septic shock. They survived when treated with nanodrugs, unlike those treated with classic forms of adenosine and vitamin E. A decrease in the production of proinflammatory cytokines was also observed after injection of nanodrugs," says Patrick Couvreur.
A promising nanodrug... but we must be patient
Both hyper-inflammatory, the symptoms of septic shock are similar to the symptoms of the most severe cases of Covid-19. The nanodrug developed by Patrick Couvreur’s team may prove effective. But it is unlikely to be available soon. “First, we need to be able to reproduce the effectiveness of these nanoparticles on an inflammatory-reaction model consecutive to Covid-19, and to make the nanodrug in large quantities for human clinical trials. Other preclinical steps are also necessary before testing it on humans, including toxicity tests on non-rodents,” says Patrick Couvreur. But results are promising for the treatment of uncontrolled inflammatory reactions in the future.
Reference;
Flavio Dormont et al., Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation. Science advances, 27 April 2020.
