Artificial radioactivity: a crucial discovery for nuclear medicine made 90 years ago
This article was originally published in L'Édition n°27.
In 1935, Irène and Frédéric Joliot-Curie were awarded the Nobel Prize in Chemistry for their discovery of artificial radioactivity. By becoming the first to produce radioactive elements, the two scientists paved the way for them to be used in numerous ways, particularly in the field of medicine. Ninety years on, teams at Université Paris-Saclay continue to enrich this heritage by developing new molecules to diagnose or treat diseases such as cancer.
"I don't remember a thing about the discovery of artificial radioactivity," admits Hélène Langevin-Joliot, Irène and Frédéric Joliot-Curie's daughter. But "I remember seeing my parents in my mother's study and hearing them say: 'We're getting a NobelPrize!'." That was November 1935. A month later, the couple travelled to Sweden to receive the Nobel Prize in Chemistry for their work on artificial radioactivity. "It was like the last piece of a puzzle pieced together over years of research into radioactivity," says Hélène Langevin-Joliot. A puzzle to which her grandparents, Marie and Pierre Curie, had already made crucial contributions, winning two Nobel Prizes in 1903 and 1911.
At the time, following these discoveries, research into radioactivity was going well, but was not without its difficulties. "It's important to understand that there was a conceptual blockage in relation to the idea that transmutation [the transformation of an atomic nucleus] could produce not a stable atom but a radioactive one. It was unthinkable," continues the former nuclear physics researcher, speaking at an event organised by the Laboratory of the Physics of the two Infinities — Irène Joliot-Curie (IJCLab - Univ. Paris-Saclay/NationalCentre for Scientific Research, CNRS/Univ. Paris-Cité) for the 90th anniversary of the discovery of artificial radioactivity.
Yet, this was the feat that her parents, Irène and Frédéric Joliot-Curie, achieved in January 1934. Using aluminium foil and a source of polonium, the two scientists obtained phosphorus-30, then called "radiophosphorus". This became the first artificially produced radioactive nucleus. "Radioactivity remained a property exclusively associated with some thirty naturally occurring bodies. The artificial creation of radioelements opens up a new field in the science of radioactivity," declared Irène Joliot-Curie in her speech on receiving the Nobel Prize.
Isotopes in search of stability
To understand the importance of this discovery, we need to delve into the heart of the matter. This matter is made up of atoms, themselves made up of small particles that form the nucleus ‒ protons and neutrons ‒ around which electrons gravitate. A chemical element is defined by the number of protons it has. But the number of neutrons involved varies from atom to atom. Atoms with the same number of protons but different numbers of neutrons are referred to as isotopes. Phosphorus, characterised by its 15 protons, has several isotopes, including phosphorus-30 (30P) and phosphorus-31(31P), which contain 15 and 16 neutrons, respectively.
Because of this difference in composition, not all isotopes have the same properties. Some are said to be stable: they remain unchanged for an extremely longtime. Others are unstable: because of their composition, they have a surplus of energy which they will shed by naturally decaying to produce more stable elements. That's radioactivity. In nature, there are a few radioactive atoms, such as uranium-238(238U). But the vast majority of atoms that make up ordinary matter are stable.
The half-life refers to the time it takes for half the nuclei in a radioisotope to decay. While 238U has a half-life of 4.5 billion years, it is much shorter for the majority of unstable isotopes. The half-life of 30P, for example, is no more than three minutes. "These atoms have a very short half-life, which is why we don't find them occurring naturally," explains Charles-Olivier Bacri, Director of Research in IJCLab's Physics and Health pole. By demonstrating that it was possible to produce such isotopes, the Joliot-Curies opened the door to hundreds of unknown atoms.
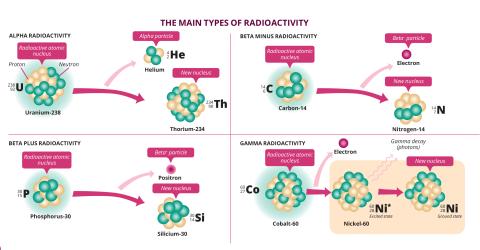
Depending on their composition, radioactive isotopes do not all decay in the same way to gain stability. This means they emit different types of radiation: alpha (α),beta (β) and gamma (γ) radiation. "α radiation is emitted by heavy nuclei with a lot of protons and neutrons. It corresponds to the emission of a helium nucleus composed of two neutrons and two protons," explains Charles-Olivier Bacri. This is particularly true of uranium-238 (238U), which decays into thorium-234 (234Th). β radiation occurs in atoms with an imbalance between the number of protons and neutrons. "When the nucleus has too many neutrons, a neutron is transformed into a proton. This is known as beta-minus decay (β-), and is accompanied by the emission of an electron (e-)." Conversely, when there are excess protons, a proton is transformed into a neutron, with the emission of a positron or anti-electron (e+). This is beta-plus decay (β+). Both α and β radioactivity involve a transformation of the atom's nucleus. However, there is another type of radiation, γ radiation, which corresponds to the radioisotope's emission of light particles or photons. "Virtually all radioactive nuclei also emit γ rays, which allow them to shed their remaining excess energy," the IJCLabphysicist explains.
Just as they do not produce the same particles, these three types of radiation have different properties and intensities. The unique feature of α radiation is that itinteracts intensely with matter, and a single sheet of paper or the thickness of your skin is enough to stop it. Less ionising, β radiation interacts less with matter, but is faster and more penetrating. Aluminium foil or glass can stop it. γ rays, on the otherhand, are the most penetrating. They can only be blocked by a thick wall of lead or concrete.
A springboard to nuclear medicine
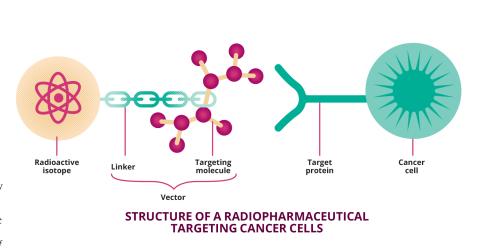
Ninety years after the Joliot-Curies' discovery, over 2,000 radioactive isotopes have been artificially created. And the uses for radioactivity have multiplied, particularly in the field of medicine, where a new speciality has even emerged: nuclear medicine, which uses radioisotopes to study the human body and diagnose or treat diseases such as cancer. To achieve this, radioactive isotopes are not used alone. They are combined with carriers – non-radioactive and generally biologicalmolecules – to make up what are known as radiopharmaceuticals. "These molecules are injected into the patient and distributed via the bloodstream to the target area that is being observed or treated," explains Charles-Olivier Bacri. Each radiopharmaceutical has a specific task. "In medical imaging, the aim is to use radioactivity to detect the target area without exposing the patient to too much energy. When it comes to treatment, it's just the opposite: the aim is to do as much 'damage' as possible in a very specific place." Each use therefore requires different radioisotopes and radiation.
In imaging, some of the most widely used tracers are β+ emitters. But various types of technological devices are used for this purpose. Gamma cameras, as their name suggests, observe γ radiation, i.e. the photons emitted by radioisotopes, to provide an image of the targeted area or organ. Positron emission tomography (PET) is based on the reaction between the positron (e+) emitted by the tracer and an electron from the surrounding matter. This results in the emission of two photons, which are identified by detectors surrounding the patient, enabling the machine to produce an accurate image of the target area, reconstructed in three dimensions.
Fluorine-18 (18F) is one of the mainradioactive markers used for imaging. In oncology, for example, it is used in the form of fluorodeoxyglucose (18F-FDG) to targetcancer cells, which require a lot of glucose, and observe tumours. But the applications of this radioisotope go far beyond that, as shown by research carried out at the Paris-Saclay Multimodal Biomedica lImaging Laboratory (BioMaps - Univ.Paris-Saclay/French Alternative Energiesand Atomic Energy Commission, CEA/CNRS/French National Institute of Health and Medical Research, Inserm), hosted bythe CEA's Frédéric Joliot Hospital Service (SHFJ) in Orsay.
50 years of producing radioisotopes on site at BioMaps
"Our job is to develop new radiopharmaceuticals, mainly for PET," says Bertrand Kuhnast, Director of SHFJ and Director of Research at BioMaps. "With 18F, we do very translational research in collaboration with other specialities. The aim is to find out how to incorporate this fluorine into molecules that are useful for imaging. For example, a biologist will tell us they're interested in a particular protein or receptor in the brain. So, we then look at how to produce an image of this target using a radiotracer."
In their research, BioMaps scientists have a remarkable asset at their disposal: their own cyclotron, a particle accelerator that produces radioactive isotopes by bombarding stable elements with protons. For the SHFJ, 2025 celebrates not only the 90th anniversary of the discovery of artificial radioactivity, but also the 50th anniversary of the installation of its first cyclotron, which arrived in 1975. Although the machine has since been dismantled, it has been replaced by two other cyclotrons at the SHFJ, making it possible to produce 18F and carbon-11 (11C), also used for imaging, on site. This is a major advantage given the radioactive half-lives of the isotopes, which are less than two hours for the former and twenty minutes for the latter.
Thanks to these facilities, the BioMaps team is working on the development of experimental radiopharmaceuticals, mainly in the fields of neurology and oncology. "Last year, we were asked to produce a radiopharmaceutical for a Phase III clinical trial involving around 100 patients," says Bertrand Kuhnast. This radiopharmaceutical, [18F] LBT-999, is no stranger to BioMaps scientists, having been developed jointly with the University of Tours and the Orphachem company, and for which a co-ownership patent was issued in 2015.
[18F] LBT-999 is a molecule that targets the dopamine transporter and aims to detect pathologies such as Parkinson's disease, characterised by the degeneration of dopaminergic neurons. Another example of a radiopharmaceutical jointly developed by BioMaps is 18F-DPA-714, amolecule targeting a protein that is over-expressed in certain brain cells in the event of neuroinflammation. "This radiopharmaceutical has been used in clinical trials on neurodegenerative diseases to try and understand when inflammation occurs," explains Bertrand Kuhnast.
While neurology and oncology are the main fields of application for molecules developed by BioMaps teams, the researchers are also venturing into other areas. A study published in 2022 explain show they carried out "radiolabelling" for a drug used in the treatment of HIV (Human Immunodeficiency Virus), dolutegravir. "When patients stop triple therapy, there is always a rebound in viral load [the amount of the virus in the blood]. This raises a number of questions about the drug's pharmacokinetics," explains the SHFJ director. How does dolutegravir interact with viral reservoirs and their environment in the body ? To find out, the BioMaps team is replacing the stable fluorine atoms present in the drug with its radioactive isotope. "We obtain a radiotracer that has exactly the same structure as the original drug, but with the difference being that we can produce an image of it." This work, which is still in progress, is being carried out as part of a European research project, RHIViera, focusing on long-term remission of HIV infection.
SIDONIE, all about isotope purity
Within IJCLab's Physics and Health team, scientists are also working to develop new radiopharmaceuticals. In recent years, however, Charles-Olivier Bacri and his colleagues have been focusing on one criterion in particular: isotope purity. "To make a radioisotope, we take a stable element and bombard it with particles to obtain the corresponding unstable atom," explains the physicist. The problem is that the stable elements used are rarely pure, and usually come in several isotopes. After irradiation, they do not produce a single radioisotope, but a cocktail of radioisotopes that are very difficult to separate. However, "additional radioisotopes are potentially dangerous for patients, notably because they expose people to an additional dose of radioactivity. Our idea was to bombard just one stable isotope, thus reducing the variety of radioisotopes produced."
Thanks to his experience as a researcher in fundamental physics, Charles-Olivier Bacri didn't have to look far to find the resource in question. In fact, he found it just next door, in the former Centre for nuclear and mass spectroscopy (In French, CSNSM), which houses a high-quality isotope separator that is the only one of its kind in Europe: SIDONIE. Commissioned in 1969 and still in operation today, this facility is capable of achieving purification rates in excess of 99.9%. It has become the focal point of the PRISM project (In French, Production d’Isotopes et Séparation pour le Médical ‒ Isotope production and separation for medical purposes). To test the process, Charles-Olivier Bacri and his team set their sights on one radioisotope in particular: terbium-155 (155Tb). "Terbium is nicknamed the SwissArmy Knife because it has four radioisotopesthat could potentially be useful for medicalpurposes. 149Tb and 161Tb are of interest forradiotherapy, while 152Tb and 155Tb can beused for imaging." The problem is that 155Tb is mainly made from gadolinium-155 (155Gd), which is commercially available but has a purity of around 92%, which has been shown to be insufficient for the production of terbium for medical use.
Thanks to SIDONIE and its separation power, the IJCLab team and the engineers in charge of the machine were able to achieve 99.98% purification of 155Gd atoms, enabling the production of 155Tb with a purity that is, in theory, more than sufficient. "The idea was to get a proof of concept. The problem is that it's an expensive process, providing small quantities of the atoms we need." So, the scientists decided to go one step further. What is the optimum level of gadolinium purity required for 155Tb to be used for medical purposes? "We know that 92% purity is not enough, but 99.98% may be far too much," explains Charles-Olivier Bacri. Through a study that is still ongoing, his team is attempting to analyse what happens when the purity of 155Gd (and therefore that of 155Tb) deteriorates, by adding 156Gd. "If the optimum level is 94%, this should be reproducible. If it's 98%, it'll be more difficult. That means we'd have to invest more to get a separator that's almost as efficient as SIDONIE."
The issue of radioisotope purity doesn't just affect the health of the patients. 156Tb has the disadvantage that it emits γ radiation with an energy close to 2 million electronvolts (MeV), compared with around 0.17 MeV for 155Tb. In addition to exposing the patient to a potentially harmful dose, this is likely to pollute the observations made using this imaging. How far can we go without polluting the image? That's what scientists have decided to investigate in another study. By mixing 155Tb and 156Tb and using a plastic box simulating a mouse, they're observing how the radioisotope 156 pollutes the image. "After a while, if the image is too polluted, there's no point in doing it anymore, even if the patient is tolerating the dose very well." Based on these two criteria ‒ dosimetry and image pollution ‒ the team hopes to determine the optimum purity for using 155Tb in the best possible conditions.
Combining diagnosis and treatment with radioisotopes
With such a versatile Swiss Army Knife in their pocket, Charles-Olivier Bacri and his colleagues can't just leave it at that. Using terbium radioisotopes, they are working to develop radiopharmaceuticals that can be used for imaging, radiotherapy or even both. This is what we call theranostics, which consists of combining diagnosis and treatment. Iodine-131 (131I), used to treat thyroid cancer, is a pioneering example. This radioisotope has the advantage of emitting β- and γ radiation, which is useful for both observing and treating thyroid tumours. With terbium, "we have four radioisotopes that can becoupled in pairs, one for imaging, the other for treatment. Since they all have the same chemistry and very similar masses, they'll have the same dynamics. We believe these would be very useful components for theranostics."
At IJCLab, as at BioMaps, the development of radiopharmaceuticals is a complex exercise that involves much more than just identifying isotopes of interest. We also need to find a way to combine them and ensure they target the right location in the body. This is the role of the vectors incorporated into radiopharmaceuticals.This could be a simple peptide targeting a receptor present in diseased cells, or a more complex vector, such as an antibody. "Antibodies bind to specific antigens. By using them, it's possible to target a tumourmore precisely", explains the physicist. Developing such radiopharmaceuticals, however, is challenging. Antibodies have a distribution time in the body of several days or even weeks. It is therefore necessary to use radioisotopes with a half-life that is compatible and long enough to persist throughout the entire process, but without delivering an un necessarydose of radiation to the body. There is also the chemical challenge of binding the radioisotope to the antibody.
Although research is still ongoing in this field, it is opening up important perspectives, involving the observation of new phenomena and development of new treatments using radioisotopes, and also achieving increasingly personalised nuclear medicine. It would then be possible to adapt the dose delivered to the specific characteristics of each patient, so as to treat them as effectively as possible, while limiting the side effects associated with radioactivity.
References :
- Curie and Joliot-Curie Association :
https://curie-joliotcurie.fr/en/home/ - Lavisse et al., Increased microglial activation in patients with Parkinson disease using [18F]-DPA714 TSPO PET imaging, Parkinsonism and Related Disorders 2021.
- Huvelle et al., Improved automated radiosynthesis of [18F]Dolutegravir: Toward clinical applications, ACS Omega 2024.
- Köster et al., Electromagnetic isotope separation of gadolinium isotopes for the production of 152,155Tb for radiopharmaceutical applications, Nuclear Instruments and Methods in Physics Research Section B 2020.
- Bouteculet et al., First production of pure 155Gd targets and 155Gd(p,x)155Tb, 156Tb cross-section measurements, Applied Radiation and Isotopes 2024.

This article was originally published in L'Édition n°27.
Find out more about the journal in digital version here and on Calameo.
For more articles and topics, subscribe to L'Édition and receive future issues: