ICARE: Mapping cells in the lungs targeted by SARS-CoV-2
In the midst of the health crisis caused by SARS-CoV-2, the University’s research laboratories will not be beaten and have thus been even more committed to fighting the virus. The Icare study, led by Isabelle Schwartz of the Virology and Molecular Immunology laboratory (VIM - Université Paris-Saclay, INRAE, UVSQ), is among the projects selected by the ANR (French National Agency for Research) following their Covid-19 flash appeal.
The objective of the Icare study is to create a map, both of the viral infection and the associated cellular response. Both innovative and original, they use strategies for keeping transplant lungs alive. By infecting these lungs with SARS-CoV-2, researchers are trying to study the initial stages of the infection on a complete organ.
The laboratory’s original collaboration
Before the Covid-19 epidemic, Isabelle Schwartz was studying interactions between dendritic cells, immune system cells, and different types of viruses, in particular ’flu and other animal or zoonotic viruses. Recently, several clinician-researchers specialising in lung transplants joined her team from the Université de Versailles - Saint-Quentin-en-Yvelines and from the Foch de Suresnes hospital. “Together, we launched a study of immunomodulation in lung transplants. The idea was to target dendritic cells in the transplant, using different strategies, to improve the chances of the transplant’s success”, explains the VIM researcher.
The Icare study exploits this dual expertise. The strength and originality of the project lies in the combination of the surgical team from Foch hospital, who bring expertise in keeping the lungs alive before transplantation and the expertise of the immunologist and virologist researchers from the VIM laboratory in lung immune cells, controlling infections under laboratory conditions and the biology of coronaviruses.
A project that is both ambitious and meticulous
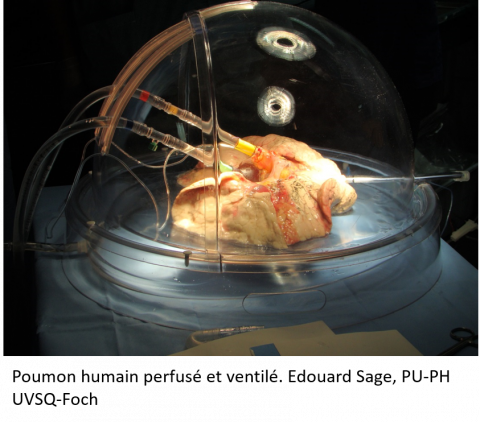
Icare involves a very complex organisational process. Firstly, the surgical team removes a human lung that has not been accepted for transplant. It is then transferred to the VIM laboratory, installed in a Level 3 confined environment and placed on artificial respiration and circulation. Once the lung is stable, the surgical team, assisted by the laboratory’s virologists, infect it with SARS-CoV-2 under very tightly controlled conditions.
Ten hours later, another team, made up of immunologists take over. They take different biopsies of the lung and sort the cell populations before carrying out RNA sequencing on unique cells. This sequencing is combined with imaging analyses of the biopsies of the infected lung, in order to identify cells that are receptive to the virus and those that respond. “We place the biopsies in a culture a little longer to increase the viral infection period. In the end, the experiments last around 35 consecutive hours, with two teams, one following the other”, explains Isabelle Schwartz.
All the analysis carried out involves several collaborations, in particular with the @Bridge platform of the Animal Genetics and Integrative Biology laboratory (GABI – Université Paris-Saclay, INRAE, AgroParisTech) for microgenomic analysis, with the Emerg’in infrastructure for the imaging and with bioinformaticians from the VIM laboratory and the Marseille-Luminy Immunology Centre (CIML – Université Aix Marseille, CNRS, Inserm).
A frenzy of research
Since the appearance of SARS-CoV-2, research in biology has focused on this virus and an international effort is under way. Hundreds of scientific articles are published each month on the subject. “Results are generated with much international competition. It is important to identify original research approaches in this current frenzy, in order to produce information that is relevant to the biology of this new virus and find ways of countering the infection and its effects”, says Isabelle Schwartz.
Maisonnasse et al, Mucosal Immunol. 2015 Nov 4. doi: 10.1038/mi.2015.105.
Sage et al, Eur J Cardiothorac Surg. 2014 Nov;46(5):794-9. doi: 10.1093/ejcts/ezu245.
Delmas et al, Nature. 1992 Jun 4;357(6377):417-20. doi: 10.1038/357417a0.