Working towards personalised cancer treatment
(This article was originally published in L'Édition No.17)
The evolution of cancer screening as well as the therapeutic interventions provided have gone hand in hand with advances in science. Before the study of radioactivity or discoveries in pharmacology gave rise to radiotherapy and chemotherapy, the first surgical treatments were limited to localized and less advanced cancers. Today, advances in genomics and immunology are paving the way for treatment to be tailored to the specific needs of each patient.
Each cancer is unique and the genetic mechanisms specific to each patient influence the cancer’s appearance, evolution and response to various treatments. Based on this, the PRISM research centre, which is accredited as a national centre for precision medicine in oncology, is involved in the development of new practices and more effective treatments.
Modelling cancer
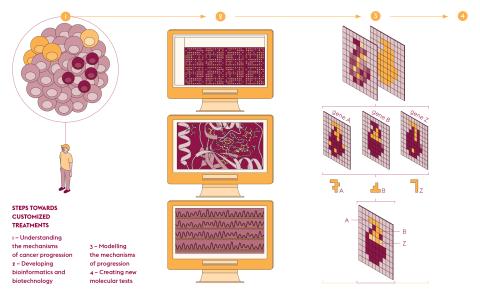
Fabrice André, who is in charge of the Molecular Predictors and New Targets in Oncology unit (PMNCO – Univ. Paris-Saclay, Gustave Roussy, Inserm) and joint manager of PRISM, sums up its objective. “Today, the sequencing of all genes leads to the identification of therapeutic targets for approximately 20% of cancer cases. We want to increase the number of patients receiving the most appropriate treatment as soon as possible.” To extend these therapeutic possibilities to as many people as possible, PRISM models each cancer using artificial intelligence to structure and interpret data from large-scale clinical trials. As Fabrice André explains, “This identity card establishes the specific molecular and cellular mechanisms of each patient. These markers predict the risk of recurrence, the effectiveness of different treatments and the occurrence of adverse effects.” And if resistance occurs, this information quickly leads to other treatments.
In the PMNCO laboratory, Fabrice André’s team has already identified markers that include, for example, a mutation deregulating the expression of the TRIB3 gene, which induces resistance to certain drugs in breast cancer. There is also the role of the HLF gene, which is involved in immune response and which could be at the root of the loss in immunity after chemotherapy.
Predicting the effectiveness of immunotherapies
Immunotherapy is among the treatments most likely to benefit from what personalised medicine can offer. Rather than targeting cancer cells directly, the treatment stimulates the immune system’s anti-tumour response. Gains in survival rates have already been shown for more than twenty-five different types of cancer. Immunotherapies also offer useful alternatives for cancers which are resistant to other treatments, as well as for patients in relapse.
However, this approach does have its limits. Immunotherapies are much better tolerated than chemotherapies, but they still generate side effects of an autoimmune nature which can be severe. In addition, some patients who were at first receptive go on to develop resistances. However, as Laurence Zitvogel, director of the Tumour Immunology and Anti-cancer Immunotherapy Unit (ITIC – Univ. Paris- Saclay, Gustave Roussy, Inserm) explains, “In some patients, the cancer regresses and sometimes disappears completely and permanently. Now the goal is to increase the number of patients who could benefit from these treatments. To achieve this, we need to understand why it does not work with some patients and develop combinations which will optimise the effect.”
To identify the immunological profile of each tumour, Professor Zitvogel’s team is using the ‘in sitro’ approach, which is a combination of ‘in situ’ and ‘in vitro’. It is ‘in situ’ because the tissues analysed come from patients who have already received immunotherapy. It is ‘in vitro’ because these samples are then stimulated using a variety of immunomodulators. The identification of the molecules released by the tumour cells then provides valuable information on the effects of different combinations of immunotherapies, as well as possible resistance. These results are a first step towards the development of predictive tests for the effectiveness of different treatment options.
Visualising gene activity
The development of high throughput DNA sequencing has propelled molecular biology into the field of data sciences and as a result has accelerated its computerization. The need to better visualise information from gene expression reads led to the creation of MULTILAYER. This open-source software has been developed by Marco Mendoza-Parra, a researcher at the Metabolic Genomics laboratory of Genoscope (Univ. Paris-Saclay, Univ. d’Évry, CNRS, CEA). “Our tool makes it possible to better exploit data on gene activity and their location,” he explains. This type of readout, known as “spatial transcriptomics”, is derived from the capture of messenger RNA by a large number of DNA probes, the positions of which are mapped.
By entrusting the processing of this information to a machine learning program, the software associates the gene activity with the spatial information of the tissue studied. The images produced are made up of “gexels”, a combination of genes and pixels, with a resolution of 100 micrometres, i.e. about ten cells. “Using this approach, information is shown as a continuum, which is totally new,” explains Marco Mendoza-Parra. “Nearby gexels share common features and provide a functional mapping of gene expression.”
This technology is very expensive at the moment due to the high throughput sequencing and the use of DNA probes. In order to overcome this barrier and provide more flexibility, Marco Mendoza-Parra is hoping to move beyond computer analysis by producing the DNA chips needed for these experiments. This new type of visualisation will help to decipher how genes are expressed within tissue complexity and ultimately help to unravel the specific mechanisms of certain pathologies.
Reducing side effects
Understanding the mechanisms of gene expression also provides useful information for tailoring cancer treatments to individual patients. The large doses of X-rays which pass through the tissue to the tumour cells during radiotherapy sometimes cause adverse reactions, which are usually transient and of low intensity. However, 10% to 15% of patients experience much more severe and long-lasting effects. “Healthy tissues surrounding the tumour are also irradiated, including blood vessels, supporting tissues and nearby organs,” explains Michèle Martin from the Laboratory of Genomics and Radiobiology of Keratinopoiesis at the Institute of Cellular and Molecular Radiobiology (IRCM – Univ. Paris-Saclay, Inserm, CEA, Univ. de Paris). “These complications include hypoplasia, a loss of tissue which can lead to necrosis, or conversely hyperplasias, where the tissue reacts and leads to fibrosis.”
Genetic pathways have been studied to discern what distinguishes radiosensitive patients from others, but so far no specific gene has been identified. “We’ve chosen a different approach, because for us this susceptibility is multiparametric in that it concerns a set of genes,” explains Michèle Martin. “We’re consequently interested in transcriptomes.” The transcriptome approach takes into account the full range of RNAs, whether they code for proteins or play a role in regulatory mechanisms without being translated (non-coding RNAs).
One of the first results of this transcriptome analysis shows an as yet unsuspected alteration of the NFATC2 gene. NFATC2 is involved in apoptosis and cell multiplication. It undergoes a repression of its expression in radiation-sensitive patients, associated with a process of hypermethylation, a common epigenetic mechanism. For Michèle Martin, “This upstream research aims to lead to clinical tests of radiosensitivity, which are being eagerly awaited by radiotherapists.”
Cancer is a complex disease with often very serious consequences. Beating it completely seems out of reach for the time being. However, the current expansion in personalised medicine is revolutionising treatment options and increasing the chances of remission for a growing number of patients.
Publications
- https://www.gustaveroussy.fr/fr/prism-ifi
- Agathe Dubuisson, et al. Immunodynamics of explanted human tumors for immuno-oncology. EMBO Molecular Medicine, 13, 2021.
- Julien Moehlin, et al. Inferring biologically relevant molecular tissue substructures by agglomerative clustering of digitized spatial transcriptomes with multilayer. Cell Systems, 12, 694-705, 2021.
- Joshua Dulong, et al. NFATC2 Modulates Radiation Sensitivity in Dermal Fibroblasts From Patients With Severe Side Effects of Radiotherapy. Frontiers in Oncology, 10, 2020
Sound and light in the fight against
Methods of diagnosing and treating cancer are constantly evolving towards less invasive techniques with fewer side effects. Today, polarised light is used to detect certain tumours. In the very near future, computer simulation will extend the use of focused ultrasound.
The earlier a cancer is detected, the more likely it is to be cured. This is especially true for skin cancers, and melanoma in particular whose tumours metastasise rapidly. However, the current detection technique, which is based on visual observation of suspicious skin patches, is 80% effective. As a result, one in five tumours is not detected in time. Élise Colin-Koeniguer, a research engineer at ONERA Palaiseau and co-founder of the start-up ITAE Medical Research is developing an innovative early diagnosis system. “The instrument operates by employing the principle of dynamic speckle which has been used for many years to observe microvascularisation,” explains Élise Colin-Koeniguer. “Until now, this technique has not been used to detect tumours because of its limited ability to penetrate the skin.” The term “speckle” refers to the moving spots which can be seen when a laser is shone on a surface. When projected onto the skin, some photons are reflected while others penetrate more deeply.
Detecting tumours under the skin
“Using the polarisation properties of light, we neutralise the surface response to access information deeper down,” continues Élise Colin-Koeniguer. This information is about the movement of blood in the capillaries which supply the tissues. The unusual metabolism of cancer cells leads to the creation of a network of blood vessels to supply them with nutrients and oxygen. “Together with our colleagues from the Institute of Pharmacology and Structural Biology (IPBS) in Toulouse, we’ve shown that our instrument detects this microvascularisation from the first day a tumour appears. There are no other validated systems which provide such early detection as far as I know.” Several working prototypes are currently on loan to partners. “Six devices are currently in circulation and we’ve had almost no negative feedback on their use,” points out Élise Colin-Koeniguer. “The results are still being analysed, but we’ve already proven the viability and reliability of the technique.”
The next step is to market the device to hospital laboratories. The process is also attracting interest beyond oncology, with offers of collaborations in the area of controlling the vascularisation of organs intended for transplantation. Eventually, approval of the product by the health authorities will unlock the coverage of associated procedures which will allow dermatologists in public practice to access this technology.
Ultrasound which heals
The medical use of ultrasound began in the 1970s with ultrasound scanning, before finding other therapeutic applications like the destruction of blood clots and tumours. Today, High Intensity Focused Ultrasound (HIFU) treatment makes use of the properties of acoustic waves which are focused by “electronic lenses” in the same way that a magnifying glass focuses light rays. “The input from the different elements of the probe must arrive at the same time at the focal point. The energy is then concentrated in an area the size of a grain of rice,” explains Sylvain Chatillon, from the Systems and Technology Integration Laboratory (LIST – Univ. Paris-Saclay, CEA). The targeted tissues absorb the energy of the waves, causing them to heat up and the cells to be destroyed.
The safety of low-intensity ultrasound gives these applications the status of minimally invasive therapies: collateral effects on neighbouring organs and/or on healthy parts of the treated organ are very limited. This therapy is particularly suitable for easily accessible tumours located in soft tissue where ultrasound propagates well. “This is the case for prostate cancer, for example,” says Sylvain Chatillon. “However, it’s more difficult for the waves to travel if they encounter obstacles, as bone walls, and their focus becomes more complex.”
Working towards modular simulation
Adapting HIFU treatment to other situations means modifying the protocols and equipment currently used. However, as Sylvain Chatillon points out, “The development of new treatment protocols require very resource-intensive and time-consuming studies before they are approved by the health authorities.” In view of this, computer simulation offers a valuable aid. The CEA-List’s CIVA Healthcare software platform offers several simulation modules, including some developed in partnership with Inserm. These optimised and tested modules ease the calculation of the transmitted sound field, the local temperature rise and the evaluation of the delivered thermal energy dose, in order to estimate the contour of the produced lesion. The approach helps to take into account the many parameters involved in a treatment protocol, for the purposes of optimisation, sensitivity analysis and performance demonstration.
This approach also applies to the personalisation of treatments. As Sylvain Chatillon points out, “Variations in patient-specific biological parameters influence the effectiveness of treatments. Our substitution model, built by learning from simulation databases, considers these specificities to provide a real-time patient-dependent simulation. Ultimately, this would lead to the customisation of protocols to optimise therapeutic performance.”
These innovations increase the chances of detecting and treating cancer. They will not replace current techniques, but will complement them to offer the most effective solutions for treatment in each situation.
Publications
- https://www.itae.fr/fr/
- Sylvain Chatillon, et al. Applications of intensive HIFU simulation based on surrogate models using the CIVA HealthCare platform. Journal of Physics: Conference Series, 1761, 2020.